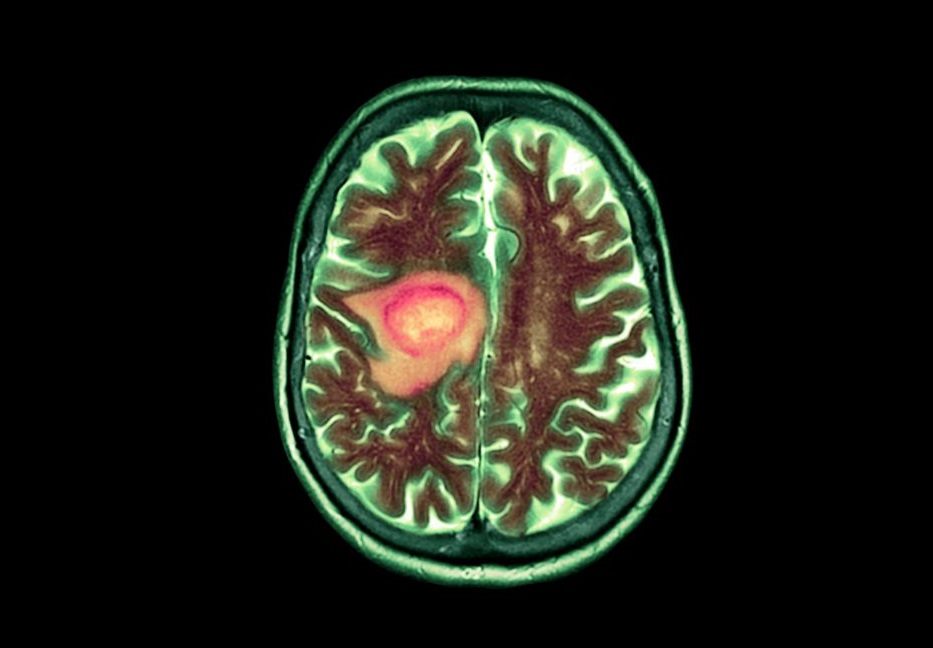
The recent approval of Vorasidenib, a new drug targeting brain tumors, has sparked intense debate within the medical community. Initially hailed as a breakthrough treatment for gliomas, the drug’s efficacy and ethical considerations are now being questioned by experts. Concerns have arisen regarding the clinical trial data, which some believe may not fully support the drug’s effectiveness in improving patient outcomes.
Critics argue that the accelerated approval process might have overlooked critical aspects of the drug’s performance. Several oncologists have pointed out that the progression-free survival benefits, a key measure of the drug’s success, were modest at best. This has led to doubts about whether the drug offers significant advantages over existing treatment options, especially when considering its high cost and potential side effects.
Ethical issues have also been raised about the marketing and accessibility of Vorasidenib. Advocacy groups have expressed concern that the drug is being promoted aggressively despite the lack of conclusive evidence supporting its superiority. They argue that patients and families, already facing difficult decisions, may be influenced by hopeful but potentially misleading claims.
Supporters of the drug, however, contend that Vorasidenib represents an important step forward in a field with limited treatment options. They emphasize that any progress in treating such a challenging condition should be welcomed, even if the benefits are incremental. Ongoing studies and real-world data may yet reveal a clearer picture of the drug’s impact.
As the debate continues, the case of Vorasidenib highlights the complexities of balancing hope and realism in cancer treatment. It underscores the need for rigorous evaluation of new therapies to ensure that patients receive safe, effective, and ethically marketed treatments.